Free Resources
Explore our Free Resources section to find detailed anatomical information and illustrations, videos, free PDF poster and activity sheet downloads, and more!
Featured Categories
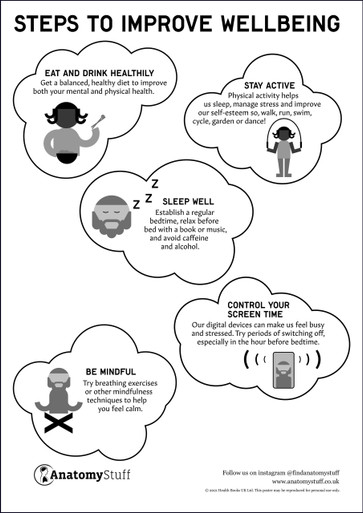
View All CategoriesPromote Health & Wellness
View All Wellness ArticlesWomen's Sexual Health
Women's sexual health is explored in our many free resources around reproduction, sexual orientation, sexual behaviour and identity, STIs, contraception, puberty and menopause amongst other female…
Hospice Coping Strategies for Families
Support for families when a loved one is in hospice care. How to deal with and accept hospice situations, how to care for loved ones approaching end-of-life care.
C-Section Aftercare – What you Need to Know
What is a C-section? C-section aftercare tips, when can I drive? All that you need to know about your C-section. Healthy eating, rest, wound care, drinking water, and exercising gently after a C-section.…
Type 2 Diabetes
Type 2 diabetes can help with lifestyle changes, support from healthcare professionals and group support. We look at how you can change your habits to support a healthier life with type 2 diabetes,…
Latest Articles
Healthcare Packs for Care Management
Spay / Neutering Excellence with Innovative Training Products
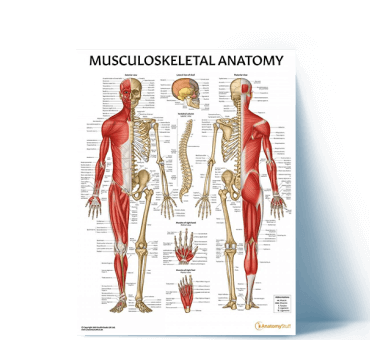
Top Musculoskeletal Resources
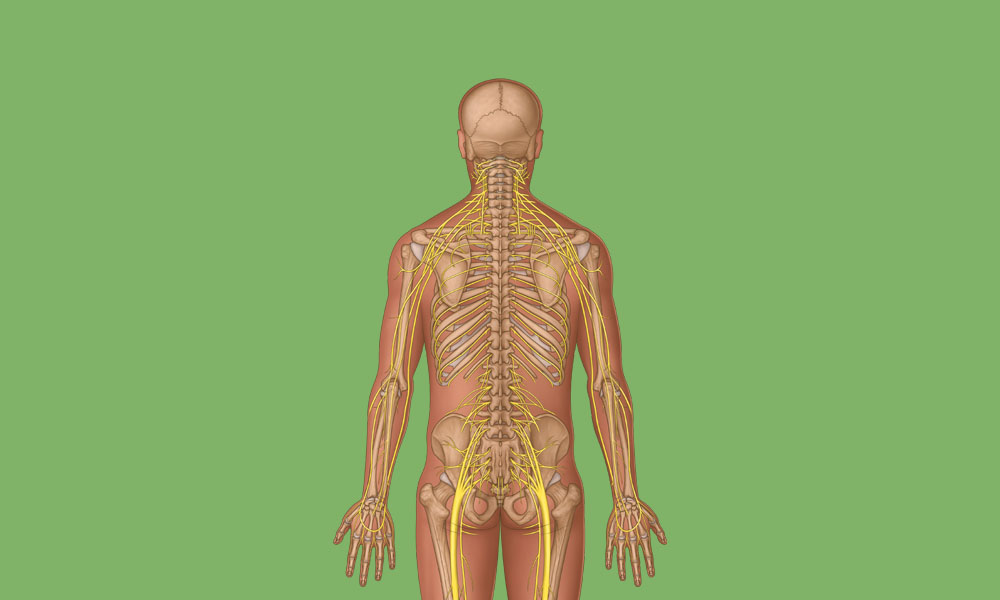
View All Musculoskeletal ArticlesSpinal Nerve Anatomy Overview
Learn about the complex anatomy of the spinal nerves and dermatomes, plus download a poster showing cervical spinal cord anatomy.
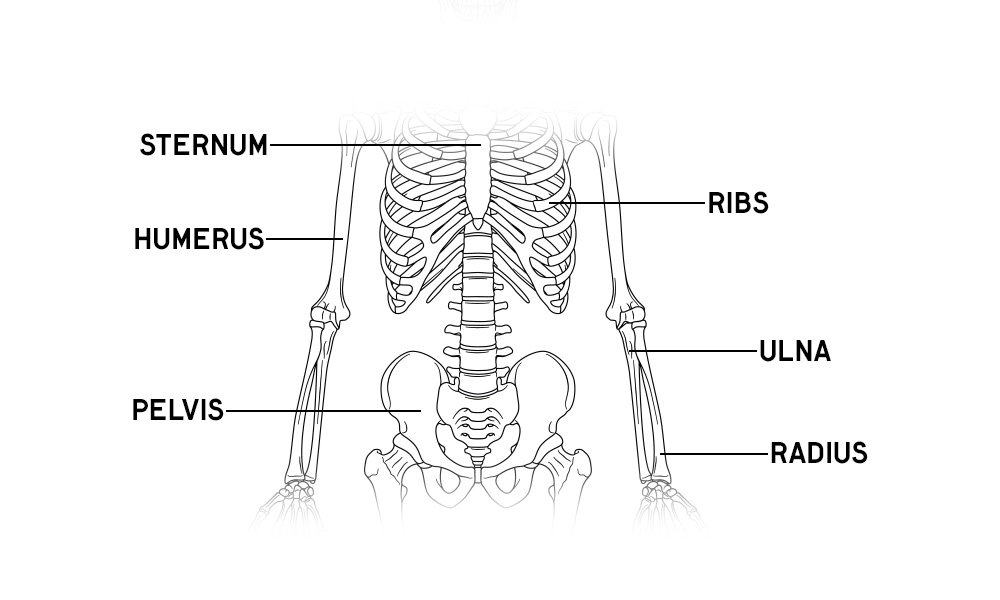
Skeleton Anatomy Overview
Download free prinatble anatomy posters and activity sheets of the human skeleton, plus learn about the bones of the body and fun facts!
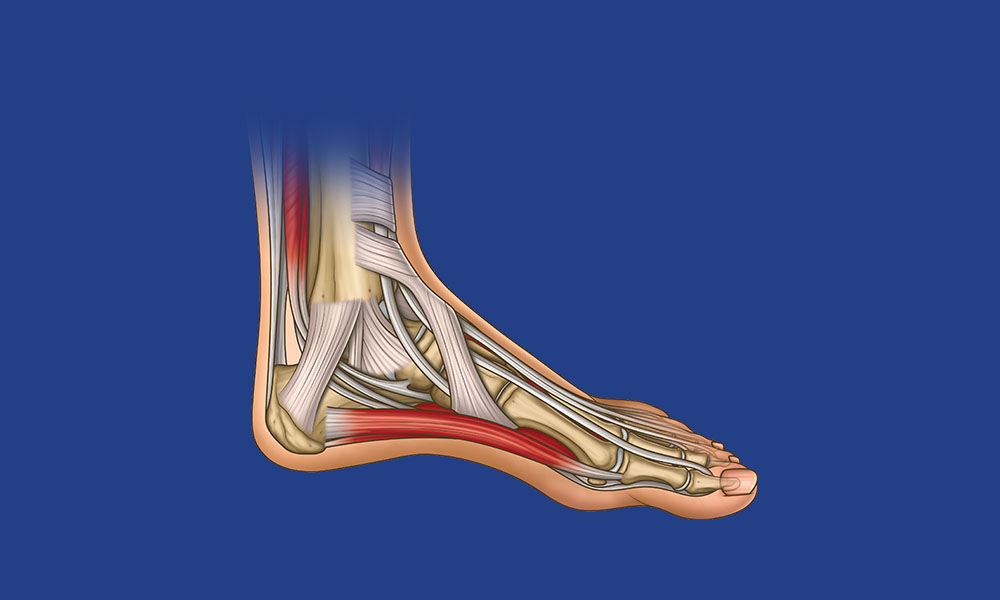
Foot & Ankle Anatomy Overview
Test your knowledge of the planes of movement of the foot, bones, muscles, ligaments and nerves. Plud get a free printable poster to learn and test your anatomy knowledge!
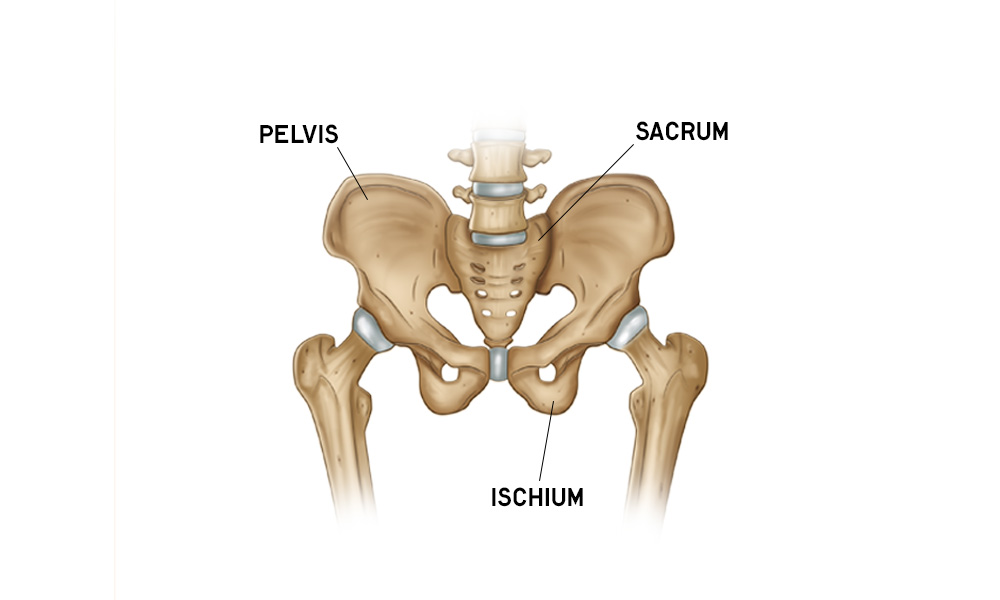
Hip Anatomy Overview
Do you want to learn about hip anatomy? Hip motion, bones, muscles, ligaments, blood vessels and nerves are all explored with free downloads. Refresh your hip anatomy knowledge.
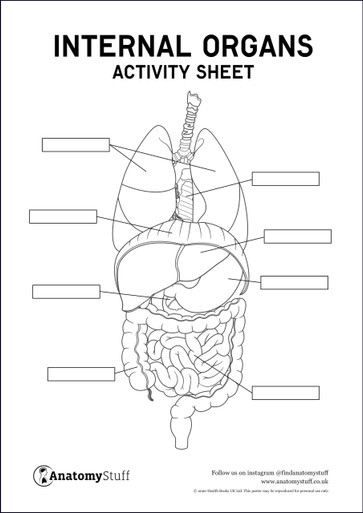
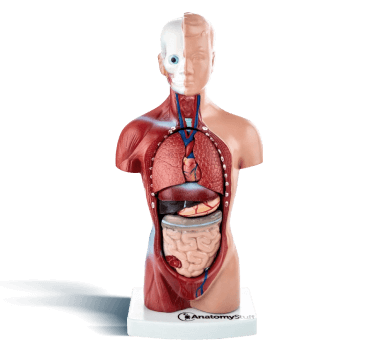
Most Popular Downloads